Abstract
BACKGROUND: Older AML patients are often NIC and face poorer prognoses. Among less intensive AML treatments, GLAS+LDAC showed improved efficacy vs LDAC alone in a Phase II randomized controlled trial (RCT). Recently, a phase I/II single arm study of VEN+LDAC showed longer overall survival (OS) in naive comparison to GLAS+LDAC. However, naïve comparisons across trials do not account for within-trial efficacy differences from control or different population characteristics. In the absence of clinical trials comparing GLAS+LDAC vs VEN+LDAC, ITC is a more robust approach, and simulated treatment comparison (STC) adjusts for between-trial differences in patient baseline characteristics. In this study, GLAS+LDAC and VEN+LDAC were indirectly compared for survival efficacy using both ITC and STC.
METHODS: Median OS and proportion of patients alive at 12 months (12-month OS) were compared for GLAS+LDAC vs VEN+LDAC using individual patient-level data (IPD) for GLAS+LDAC (n=116) and published results for VEN+LDAC (n=61). As the VEN+LDAC study lacked a control group, ITC and STC methods followed the unanchored, single-arm comparison as guided by the National Institute of Health and Care Excellence Decision Support Unit. Mean differences (MD) in median OS (months) between treatments were estimated using a modified Bucher ITC approach with 95% confidence intervals (CI). For 12-month OS, significance tests for MDs between treatments were conducted in Stata (MD with 95% CI). While ITC did not adjust for patient characteristics, using STC, GLAS+LDAC IPD were adjusted for VEN+LDAC patient baseline covariates. Full models included mutually reported covariates between trials, adjusting for GLAS+LDAC patients being older, having less denovo AML, higher bone marrow blasts, worse ECOG performance status, poorer cytogenetic risk, and more males compared to VEN+LDAC patients. STC models were estimated both with and without proportional hazards (PH) assumptions, and the best fitting models were applied.
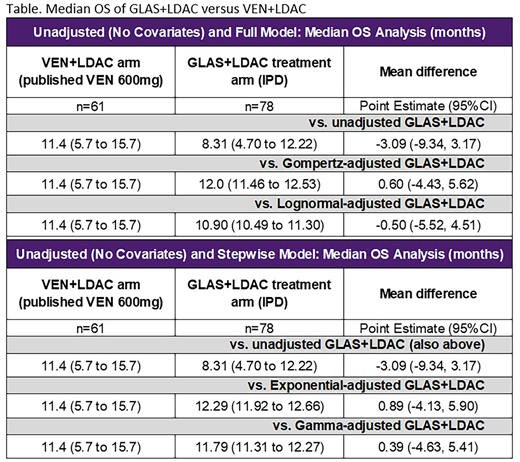
RESULTS: In the naive comparison, GLAS+LDAC had shorter median OS (8.3 months) vs VEN+LDAC (11.4 months). Similarly, 12-month OS was lower for GLAS+LDAC (39.4%) vs VEN+LDAC (45.9%). ITC found non-significant differences between GLAS+LDAC and VEN+LDAC for median OS [MD=-3.09 (-9.34, 3.17)] and 12-month OS [MD=-0.07 (-0.23, 0.10)], with numeric trends favoring VEN+LDAC (as shown in the Table). With full STC adjustment, PH models showed numeric but not statistical superiority of GLAS+LDAC vs VEN+LDAC for median OS [e.g., Gompertz-derived MD=0.60 (-4.43, 5.62)]. In contrast, full covariate models without the PH assumption estimated numeric but not statistical superiority of VEN+LDAC [e.g., lognormal-derived MD=-0.50 (-5.52, 4.51)]. Stepwise model results reflected full covariate adjustments: PH models numerically favored GLAS+LDAC over VEN+LDAC by about one month [e.g., exponential-derived MD=0.89 (-4.13, 5.90)]. Among models relaxing the PH assumption, the gamma estimate numerically favored GLAS+LDAC over VEN+LDAC [MD=0.39 (-4.63, 5.41)].
For 12-month OS, with all STC approaches, differences between GLAS+LDAC and VEN+LDAC were not statistically significant. After full covariate adjustment, slight numeric improvement with GLAS+LDAC vs VEN+LDAC was found for PH models [e.g., Gompertz-derived MD=0.04 (-0.13, 0.21)] and non-PH models [e.g., lognormal-derived MD=0.01 (-0.16, 0.17)]. Stepwise covariate models generated highly similar results (data not shown).
CONCLUSIONS: Although GLAS+LDAC and VEN+LDAC have not been directly compared in a clinical trial, ITC and STC approaches found no difference between the two treatments comparing median OS and 12-month OS. Moreover, indirect approaches may introduce less bias than naïve comparisons, as methods can include statistical testing of differences and adjusting for population baseline differences. Standard ITC, which account for within-trial improvements from placebo could not be performed due to the single-arm design of the VEN+LDAC study. Furthermore, single-arm, open-label trials such as the VEN+LDAC study may overestimate treatment effectiveness, and GLAS+LDAC estimates originated from a robustly conducted RCT. As there was no evidence of statistical differences between treatments, health care stakeholders should continue to prioritize RCT evidence generation and minimize inferences from naïve comparisons.
Forsythe:Novartis: Consultancy. Bell:Pfizer: Employment, Equity Ownership. Cappelleri:Pfizer: Employment, Equity Ownership. Chan:Pfizer: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.